Abstract
INTRODUCTION
Recent approvals in multiple myeloma (MM) treatment have focused on addressing unmet needs among patients who have failed prior therapies. Pomalidomide (POM) is an IMiD® immunomodulatory agent approved in the United States (US) by the US Food and Drug Administration in 2013 in combination with low-dose dexamethasone (Dex) for patients with MM who have received ≥ 2 prior therapies including lenalidomide and a protease inhibitor in relapsed or refractory multiple myeloma (RRMM). Due to the relatively small population with RRMM and the inherent limitations of most observational research databases, no previous study has described the real-world patterns of using POM in RRMM in the US. The purpose of this analysis was to describe the clinical characteristics, treatment patterns, and regimen sequencing of patients who received POM in a US practice setting using an enhanced electronic health records (EHR) database containing over 6000 patients with MM across the US.
METHODS
This retrospective observational study identified patients with MM (ICD-9: 203.0x or ICD-10: C90.xx) between 01/01/2011 and 05/31/2017 in the Flatiron Health electronic records database. Diagnosis of MM was confirmed through review of unstructured chart data (eg, physician notes in the EHR). The Flatiron MM provider network comprises over 265 clinics throughout the US. Patients were included if they received POM in any line of therapy and were not enrolled in a clinical trial. Patients were further categorized into 2 subgroups: POM in second-line therapy (POM 2L) or POM in third-line therapy (POM 3L). Demographic and clinical characteristics were described at baseline. Laboratory values were obtained from structured fields and were reported if available within 60 days of the date of POM therapy initiation. Regimens received in the line prior to and post-POM use were described, as was the patient disposition at the end of the study period.
RESULTS
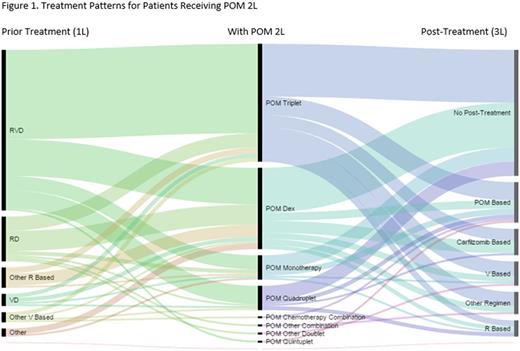
For the 572 pomalidomide patients (ALL POM) identified, mean (SD) age was 67.2 (10.5) years at the time of first exposure to POM, 50.2% were female, 61.7% white, 14.7% African American, and 23.6% other/unknown races. In the ALL POM population with available laboratory values, 457 (99.1%) had serum calcium levels of < 11 mg/dL. Of the 373 ALL POM patients with available estimated glomerular filtration rate (eGFR) data, baseline values were > 60 mL/min in 212 (56.8%), 30-60 mL/min in 115 (30.8%), < 30 mL/min with no dialysis in 37 (9.9%), and < 30 mL/min with dialysis in 9 (2.4%). In those with available International Staging System data (n = 169), 27.8% were stage I, 33.7% were stage II, and 38.5% were stage III. There were 126 patients (22.0%) who received POM 2L and 115 (20.1%) received POM 3L. Clinical characteristics were similar across subgroups, except M-Spike and free light chain ratio were higher in POM 3L. The most common POM-based regimens received in 2L were POM-Dex (POM-d; 40 [31.8%]), followed by POM-carfilzomib-Dex (PKd; 33 [26.2%]); the most common in 3L were POM-d (47 [40.9%]) and PKd (19 [16.5%]). Lenalidomide in the immediate prior line of therapy was received by 88.1% of POM 2L and 66.1% of POM 3L. In the POM 3L group, 90.4% of patients received lenalidomide in any prior line of therapy. Most patients in POM 2L received lenalidomide, bortezomib, and Dex (RVd) 1L (79 [62.7%]). Of the 126 patients in POM 2L, 39.1% received POM in any subsequent line and 49.2% were categorized as receiving no post-treatment for the following reasons: 28.6% were lost to follow-up, 7.9% were in a treatment gap (defined as a gap of > 180 days), 7.9% had died, and 4.8% were still in POM 2L treatment at the end of the study (Figure 1). Of the 115 patients in POM 3L, 34.0% received POM in any subsequent line and 53.9% were categorized as receiving no post-treatment: 16.5% were lost to follow-up, 10.4% were in a treatment gap, 24.4% had died, and 2.6% were still in POM 3L treatment at the end of the study.
CONCLUSIONS
Data from an enhanced EHR database showed that most patients who received POM in 2L or 3L treatment were exposed to lenalidomide in prior therapy. The clinical characteristics (serum calcium levels and eGFR) of POM patients also suggest that POM was used in patients with various degrees of renal impairment. With a longer follow-up time, we will be able to observe treatment patterns in later lines of therapy and determine the value newer agents have in the management of patients with MM.
Clancy: Celgene Corporation: Employment. Mouro: Celgene Corporation: Employment, Equity Ownership. Tian: Celgene: Employment. Abouzaid: Celgene Corporation: Employment, Equity Ownership, Research Funding. Richter: Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal